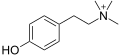
Tyramine
 Skeletal formula of tyramine | |
 Ball-and-stick model of the neutral (non-zwitterionic) form of tyramine found in the crystal structure[1] | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈtaɪrəmiːn/ TY-rə-meen |
| Other names | Tyramin; 4-Hydroxyphenethylamine; para-Tyramine; p-Tyramine; 4-Tyramine; Mydrial; Uteramin |
| ATC code |
|
| Pharmacokinetic data | |
| Metabolism | CYP2D6, flavin-containing monooxygenase 3, monoamine oxidase A, monoamine oxidase B, phenylethanolamine N-methyltransferase, dopamine β-hydroxylase, others |
| Metabolites | 4-Hydroxyphenylacetaldehyde, dopamine, N-methyltyramine, octopamine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.106 |
| Chemical and physical data | |
| Formula | C8H11NO |
| Molar mass | 137.182 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.103 g/cm3 predicted[2] |
| Melting point | 164.5 °C (328.1 °F) [3] |
| Boiling point | 206 °C (403 °F) at 25 mmHg; 166 °C at 2 mmHg[3] |
| |
| |
Tyramine (/ˈtaɪrəmiːn/ TY-rə-meen) (also spelled tyramin), also known under several other names,[note 1] is a naturally occurring trace amine derived from the amino acid tyrosine.[4] Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs).
Occurrence
[edit]Tyramine occurs widely in plants[5] and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date.
Specific foods containing considerable amounts of tyramine include:[6][7]
- Strong or aged cheeses: cheddar, Swiss, Parmesan, Stilton, Gorgonzola or blue cheeses, Camembert, feta, Muenster
- Meats that are cured, smoked, or processed: such as salami, pepperoni, dry sausages, hot dogs, bologna, bacon, corned beef, pickled or smoked fish, caviar, aged chicken livers, soups or gravies made from meat extract
- Pickled or fermented foods: sauerkraut, kimchi, tofu (especially stinky tofu), pickles, miso soup, bean curd, tempeh, sourdough breads
- Condiments: soy, shrimp, fish, miso, teriyaki, and bouillon-based sauces
- Drinks: beer (especially tap or home-brewed), vermouth, red wine, sherry, liqueurs
- Beans, vegetables, and fruits: fermented or pickled vegetables, overripe fruits
- Chocolate[8]
Scientists more and more consider tyramine in food as an aspect of safety.[9] They propose projects of regulations aimed to enact control of biogenic amines in food by various strategies, including usage of proper fermentation starters, or preventing their decarboxylase activity.[10] Some authors wrote that this has already given positive results, and tyramine content in food is now lower than it has been in the past.[11]
In plants
[edit]Mistletoe (toxic and not used by humans as a food, but historically used as a medicine).[12]
In animals
[edit]Tyramine also plays a role in animals including: In behavioral and motor functions in Caenorhabditis elegans;[13] Locusta migratoria swarming behaviour;[14] and various nervous roles in Rhipicephalus, Apis, Locusta, Periplaneta, Drosophila, Phormia, Papilio, Bombyx, Chilo, Heliothis, Mamestra, Agrotis, and Anopheles.[15]
Physical effects and pharmacology
[edit]Evidence for the presence of tyramine in the human brain has been confirmed by postmortem analysis.[16] Additionally, the possibility that tyramine acts directly as a neuromodulator was revealed by the discovery of a G protein-coupled receptor with high affinity for tyramine, called TAAR1.[17][18] The TAAR1 receptor is found in the brain, as well as peripheral tissues, including the kidneys.[19] Tyramine binds to TAAR1 as an agonist in humans.[20]
Tyramine is physiologically metabolized by monoamine oxidases (primarily MAO-A), FMO3, PNMT, DBH, and CYP2D6.[21][22][23][24][25] Human monoamine oxidase enzymes metabolize tyramine into 4-hydroxyphenylacetaldehyde.[26] If monoamine metabolism is compromised by the use of monoamine oxidase inhibitors (MAOIs) and foods high in tyramine are ingested, a hypertensive crisis can result, as tyramine also can displace stored monoamines, such as dopamine, norepinephrine, and epinephrine, from pre-synaptic vesicles. Tyramine is considered a "false neurotransmitter", as it enters noradrenergic nerve terminals and displaces large amounts of norepinephrine, which enters the blood stream and causes vasoconstriction.
Additionally, cocaine has been found to block blood pressure rise that is originally attributed to tyramine, which is explained by the blocking of adrenaline by cocaine from reabsorption to the brain.[27]
The first signs of this effect were discovered by a British pharmacist who noticed that his wife, who at the time was on MAOI medication, had severe headaches when eating cheese.[28] For this reason, it is still called the "cheese reaction" or "cheese crisis", although other foods can cause the same problem.[29]
Most processed cheeses do not contain enough tyramine to cause hypertensive effects, although some aged cheeses (such as Stilton) do.[30][31]
A large dietary intake of tyramine (or a dietary intake of tyramine while taking MAO inhibitors) can cause the tyramine pressor response, which is defined as an increase in systolic blood pressure of 30 mmHg or more. The increased release of norepinephrine (noradrenaline) from neuronal cytosol or storage vesicles is thought to cause the vasoconstriction and increased heart rate and blood pressure of the pressor response. In severe cases, adrenergic crisis can occur.[medical citation needed] Although the mechanism is unclear, tyramine ingestion also triggers migraine attacks in sensitive individuals and can even lead to stroke.[32] Vasodilation, dopamine, and circulatory factors are all implicated in the migraines. Double-blind trials suggest that the effects of tyramine on migraine may be adrenergic.[33]
Research reveals a possible link between migraines and elevated levels of tyramine. A 2007 review published in Neurological Sciences[34] presented data showing migraine and cluster diseases are characterized by an increase of circulating neurotransmitters and neuromodulators (including tyramine, octopamine, and synephrine) in the hypothalamus, amygdala, and dopaminergic system. People with migraine are over-represented among those with inadequate natural monoamine oxidase, resulting in similar problems to individuals taking MAO inhibitors. Many migraine attack triggers are high in tyramine.[35]
If one has had repeated exposure to tyramine, however, there is a decreased pressor response; tyramine is degraded to octopamine, which is subsequently packaged in synaptic vesicles with norepinephrine (noradrenaline).[citation needed] Therefore, after repeated tyramine exposure, these vesicles contain an increased amount of octopamine and a relatively reduced amount of norepinephrine. When these vesicles are secreted upon tyramine ingestion, there is a decreased pressor response, as less norepinephrine is secreted into the synapse, and octopamine does not activate alpha or beta adrenergic receptors.[medical citation needed]
When using a MAO inhibitor (MAOI), an intake of approximately 10 to 25 mg of tyramine is required for a severe reaction, compared to 6 to 10 mg for a mild reaction.[36]
Tyramine, like phenethylamine, is a monoaminergic activity enhancer (MAE) of serotonin, norepinephrine, and dopamine in addition to its catecholamine-releasing activity.[37][38][39] That is, it enhances the action potential-mediated release of these monoamine neurotransmitters.[37][38][39] The compound is active as a MAE at much lower concentrations than the concentrations at which it induces the release of catecholamines.[37][38][39] The MAE actions of tyramine and other MAEs may be mediated by TAAR1 agonism.[40][41]
Biosynthesis
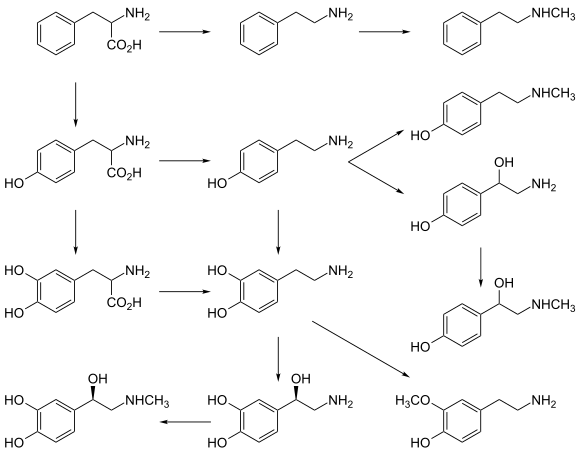
[edit]Biochemically, tyramine is produced by the decarboxylation of tyrosine via the action of the enzyme tyrosine decarboxylase.[42] Tyramine can, in turn, be converted to methylated alkaloid derivatives N-methyltyramine, N,N-dimethyltyramine (hordenine), and N,N,N-trimethyltyramine (candicine).
-
Tyramine
-
N-Methyltyramine
-
N,N-Dimethyltyramine (hordenine)
-
N,N,N-Trimethyltyramine (candicine)
In humans, tyramine is produced from tyrosine, as shown in the following diagram.
Chemistry
[edit]In the laboratory, tyramine can be synthesized in various ways, in particular by the decarboxylation of tyrosine.[43][44][45]

Society and culture
[edit]Legal status
[edit]United States
[edit]Tyramine is a Schedule I controlled substance, categorized as a hallucinogen, making it illegal to buy, sell, or possess in the state of Florida without a license at any purity level or any form whatsoever. The language in the Florida statute says tyramine is illegal in "any material, compound, mixture, or preparation that contains any quantity of [tyramine] or that contains any of [its] salts, isomers, including optical, positional, or geometric isomers, and salts of isomers, if the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation."[46]
This ban is likely the product of lawmakers overly eager to ban substituted phenethylamines, which tyramine is, in the mistaken belief that ring-substituted phenethylamines are hallucinogenic drugs like the 2C series of psychedelic substituted phenethylamines. The further banning of tyramine's optical isomers, positional isomers, or geometric isomers, and salts of isomers where they exist, means that meta-tyramine and phenylethanolamine, a substance found in every living human body, and other common, non-hallucinogenic substances are also illegal to buy, sell, or possess in Florida.[46] Given that tyramine occurs naturally in many foods and drinks (most commonly as a by-product of bacterial fermentation), e.g. wine, cheese, and chocolate, Florida's total ban on the substance may prove difficult to enforce.[47]
Notes
[edit]References
[edit]- ^ Cruickshank L, Kennedy AR, Shankland N (2013). "Tautomeric and ionisation forms of dopamine and tyramine in the solid state". J. Mol. Struct. 1051: 132–136. Bibcode:2013JMoSt1051..132C. doi:10.1016/j.molstruc.2013.08.002.
- ^ SciFinder, Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2021 ACD/Labs)
- ^ a b The Merck Index, 10th Ed. (1983), p. 1405, Rahway: Merck & Co.
- ^ "tyramine | C8H11NO". PubChem. Retrieved 8 April 2017.
- ^ T. A. Smith (1977) Phytochemistry 16 9–18.
- ^ Hall-Flavin DK (18 December 2018). "Avoid the combination of high-tyramine foods and MAOIs". Mayo Clinic.
- ^ Robinson J (21 June 2020). "Tyramine-Rich Foods As A Migraine Trigger & Low Tyramine Diet". WebMD.
- ^ "Tyramine". pubchem.ncbi.nlm.nih.gov.
- ^ Martuscelli M, Esposito L, Mastrocola D (January 2021). "Biogenic Amines' Content in Safe and Quality Food". Foods. 10 (1): 100. doi:10.3390/foods10010100. PMC 7825060. PMID 33418895.
- ^ "Scientific Opinion on risk based control of biogenic amine formation in fermented foods". EFSA Journal. 9 (10): 2393. 2011. doi:10.2903/j.efsa.2011.2393.
- ^ Finberg JP, Gillman K (2011). "Selective inhibitors of monoamine oxidase type B and the "cheese effect"". Monoamine Oxidase and their Inhibitors. International Review of Neurobiology. Vol. 100. pp. 169–190. doi:10.1016/B978-0-12-386467-3.00009-1. ISBN 978-0-12-386467-3. PMID 21971008.
- ^ "Tyramine". American Chemical Society. 19 December 2005.
- ^ Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR (April 2005). "Tyramine Functions Independently of Octopamine in the Caenorhabditis elegans Nervous System". Neuron. 46 (2). Cell Press (Elsevier BV): 247–60. doi:10.1016/j.neuron.2005.02.024. PMID 15848803. S2CID 14914393.
- ^ Ma Z, Guo X, Lei H, Li T, Hao S, Kang L (January 2015). "Octopamine and tyramine respectively regulate attractive and repulsive behavior in locust phase changes". Scientific Reports. 5 (1). Nature/Springer: 8036. Bibcode:2015NatSR...5E8036M. doi:10.1038/srep08036. PMC 5389030. PMID 25623394. S2CID 2056338.
- ^ Ohta H, Ozoe Y (2014). "Molecular Signalling, Pharmacology, and Physiology of Octopamine and Tyramine Receptors as Potential Insect Pest Control Targets". Advances in Insect Physiology. Vol. 46. Elsevier. pp. 73–166. doi:10.1016/b978-0-12-417010-0.00002-1. ISBN 978-0-12-417010-0. S2CID 80723865.
- ^ Philips SR, Rozdilsky B, Boulton AA (February 1978). "Evidence for the presence of m-tyramine, p-tyramine, tryptamine, and phenylethylamine in the rat brain and several areas of the human brain". Biological Psychiatry. 13 (1): 51–7. PMID 623853.
- ^ Navarro HA, Gilmour BP, Lewin AH (September 2006). "A rapid functional assay for the human trace amine-associated receptor 1 based on the mobilization of internal calcium". Journal of Biomolecular Screening. 11 (6): 688–93. doi:10.1177/1087057106289891. PMID 16831861.
- ^ Liberles SD, Buck LB (August 2006). "A second class of chemosensory receptors in the olfactory epithelium". Nature. 442 (7103): 645–50. Bibcode:2006Natur.442..645L. doi:10.1038/nature05066. PMID 16878137. S2CID 2864195.
- ^ Xie Z, Westmoreland SV, Miller GM (May 2008). "Modulation of monoamine transporters by common biogenic amines via trace amine-associated receptor 1 and monoamine autoreceptors in human embryonic kidney 293 cells and brain synaptosomes". The Journal of Pharmacology and Experimental Therapeutics. 325 (2): 629–640. doi:10.1124/jpet.107.135079. PMID 18310473. S2CID 178180.
- ^ Khan MZ, Nawaz W (October 2016). "The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system". Biomedicine & Pharmacotherapy. 83: 439–449. doi:10.1016/j.biopha.2016.07.002. PMID 27424325.
- ^ "Trimethylamine monooxygenase (Homo sapiens)". BRENDA. Technische Universität Braunschweig. July 2016. Retrieved 18 September 2016.
- ^ Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–87. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - ^ a b Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ a b Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ a b Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ "4-Hydroxyphenylacetaldehyde". Human Metabolome Database – Version 4.0. University of Alberta. 23 July 2019. Retrieved 8 August 2019.
- ^ Bynum W (27 April 2013). "REVIEW --- Books: What Sets Your Heart Pounding". Wall Street Journal. p. C.6. ProQuest 1346292101.
- ^ Sathyanarayana Rao TS, Yeragani VK (January 2009). "Hypertensive crisis and cheese". Indian Journal of Psychiatry. 51 (1): 65–6. doi:10.4103/0019-5545.44910. PMC 2738414. PMID 19742203.
- ^ Mitchell ES (2004). Triggle DJ (ed.). "Drugs, The Straight Facts: Antidepressants" (PDF). Chelsea House Publishers. pp. 30–31. Archived from the original (PDF) on 14 February 2017. Retrieved 6 October 2022.
- ^ Stahl SM, Felker A (October 2008). "Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants". CNS Spectrums. 13 (10): 855–870. doi:10.1017/S1092852900016965. PMID 18955941. S2CID 6118722.
- ^ "Tyramine-restricted Diet" (PDF). W.B. Saunders Company. 1998. Archived from the original (PDF) on 13 May 2014.
- ^ "Tyramine". Biochemistry. Encyclopedia Britannica. Retrieved 12 November 2021.
- ^ Ghose K, Coppen A, Carrol D (May 1977). "Intravenous tyramine response in migraine before and during treatment with indoramin". British Medical Journal. 1 (6070): 1191–3. doi:10.1136/bmj.1.6070.1191. PMC 1606859. PMID 324566.
- ^ D'Andrea G, Nordera GP, Perini F, Allais G, Granella F (May 2007). "Biochemistry of neuromodulation in primary headaches: focus on anomalies of tyrosine metabolism". Neurological Sciences. 28 (S2): S94-6. doi:10.1007/s10072-007-0758-4. PMID 17508188. S2CID 1548732.
- ^ "Headache Sufferer's Diet | National Headache Foundation". National Headache Foundation. Archived from the original on 2 July 2017. Retrieved 8 April 2017.
- ^ McCabe BJ (August 1986). "Dietary tyramine and other pressor amines in MAOI regimens: a review". Journal of the American Dietetic Association. 86 (8): 1059–64. doi:10.1016/S0002-8223(21)04074-8. PMID 3525654. S2CID 902921.
- ^ a b c Shimazu S, Miklya I (May 2004). "Pharmacological studies with endogenous enhancer substances: beta-phenylethylamine, tryptamine, and their synthetic derivatives". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 28 (3): 421–427. doi:10.1016/j.pnpbp.2003.11.016. PMID 15093948. S2CID 37564231.
- ^ a b c Knoll J (August 2003). "Enhancer regulation/endogenous and synthetic enhancer compounds: a neurochemical concept of the innate and acquired drives". Neurochem Res. 28 (8): 1275–1297. doi:10.1023/a:1024224311289. PMID 12834268.
- ^ a b c Knoll J, Miklya I, Knoll B, Markó R, Rácz D (1996). "Phenylethylamine and tyramine are mixed-acting sympathomimetic amines in the brain". Life Sci. 58 (23): 2101–2114. doi:10.1016/0024-3205(96)00204-4. PMID 8649195.
- ^ Harsing LG, Knoll J, Miklya I (August 2022). "Enhancer Regulation of Dopaminergic Neurochemical Transmission in the Striatum". Int J Mol Sci. 23 (15): 8543. doi:10.3390/ijms23158543. PMC 9369307. PMID 35955676.
- ^ Harsing LG, Timar J, Miklya I (August 2023). "Striking Neurochemical and Behavioral Differences in the Mode of Action of Selegiline and Rasagiline". Int J Mol Sci. 24 (17): 13334. doi:10.3390/ijms241713334. PMC 10487936. PMID 37686140.
- ^ "Tyrosine metabolism - Reference pathway". Kyoto Encyclopedia of Genes and Genomes (KEGG). Archived from the original on 26 July 2019. Retrieved 3 October 2011.
- ^ Barger G (1909). "CXXVII.?Isolation and synthesis of p-hydroxyphenylethylamine, an active principle of ergot soluble in water". J. Chem. Soc. 95: 1123–1128. doi:10.1039/ct9099501123.
- ^ Waser E (1925). "Untersuchungen in der Phenylalanin-Reihe VI. Decarboxylierung des Tyrosins und des Leucins". Helvetica Chimica Acta. 8: 758–773. doi:10.1002/hlca.192500801106.
- ^ Buck JS (1933). "Reduction of Hydroxymandelonitriles. A New Synthesis of Tyramine". Journal of the American Chemical Society. 55 (8): 3388–3390. doi:10.1021/ja01335a058.
- ^ a b "Statutes & Constitution :View Statutes : Online Sunshine". leg.state.fl.us. Retrieved 3 April 2019.
- ^ Suzzi G, Torriani S (18 May 2015). "Editorial: Biogenic amines in foods". Frontiers in Microbiology. 6: 472. doi:10.3389/fmicb.2015.00472. PMC 4435245. PMID 26042107.